Most scientists of the late nineteenth
century accepted the idea that the chemical elements consist of atoms, but they
knew almost nothing about the atoms themselves. One clue was the discovery that
all atoms contain electrons. Since electrons carry negative charges whereas
atoms are neutral, positively charged matter of some kind must be present in
atoms. But what kind? And arranged in what way?
One suggestion, made by the British
physicist J. J. Thomson in 1898, was that atoms are just positively charged
lumps of matter with electrons embedded in them, like raisins in a fruitcake
(Fig. 4.1). Because Thomson had played an important role in discovering the
electron, his idea was taken seriously. But the real atom turned out to be
quite different.
Figure4.1 The Thomson model of the atom. The
Rutherford scattering experiment showed it to be incorrect.
The most direct way to find out what is
inside a fruitcake is to poke a finger into it, which is essentially what Hans
Geiger and Ernest Marsden did in 1911. At the suggestion of Ernest Rutherford,
they used as probes the fast alpha particles emitted by certain radioactive
elements. Alpha particles are helium atoms that have lost two electrons each,
leaving them with a charge of +2e.
Geiger and Marsden placed a sample of an
alpha-emitting substance behind a lead screen with a small hole in it, as in
Fig. 4.2, so that a narrow beam of alpha particles was produced. This beam was
directed at a thin gold foil. A zinc sulfide screen, which gives off a visible
flash of light when struck by an alpha particle, was set on the other side of
the foil with a microscope to see the flashes.
Figure 4.2 The Rutherford scattering
experiment.
It was expected that the alpha particles
would go right through the foil with hardly any deflection. This follows from
the Thomson model, in which the electric charge inside an atom is assumed to be
uniformly spread through its volume. With only weak electric forces exerted on
them, alpha particles that pass through a thin foil ought to be deflected only
slightly, 1° or less.
What Geiger and Marsden actually found was
that although most of the alpha particles indeed were not deviated by much, a
few were scattered through very large angles. Some were even scattered in the
backward direction. As Rutherford remarked, “It was as incredible as if you fired
a 15-inch shell at a piece of tissue paper and it came back and hit you.”
Alpha particles are relatively heavy (almost
8000 electron masses) and those used in this experiment had high speeds
(typically 2 X 107 m/s), so it was clear that powerful forces were
needed to cause such marked deflections. The only way to explain the results,
Rutherford found, was to picture an atom as being composed of a tiny nucleus in
which its positive charge and nearly all its mass are concentrated, with the
electrons some distance away (Fig. 4.3). With an atom being largely empty
space, it is easy to see why most alpha particles go right through a thin foil.
However, when an alpha particle happens to come near a nucleus, the intense
electric field there scatters it through a large angle. The atomic electrons,
being so light, do not appreciably affect the alpha particles.
Figure 4.3 The Rutherford model of the atom.
The experiments of Geiger and Marsden and
later work of a similar kind also supplied information about the nuclei of the
atoms that composed the various target foils. The deflection of an alpha
particle when it passes near a nucleus depends on the magnitude of the nuclear
charge. Comparing the relative scattering of alpha particles by different foils
thus provides a way to find the nuclear charges of the atoms involved.
All the atoms of any one element turned out
to have the same unique nuclear charge, and this charge increased regularly
from element to element in the periodic table. The nuclear charges always turned
out to be multiples of +e; the number Z of unit positive charges in the nuclei
of an element is today called the atomic number of the element. We know now
that protons, each with a charge +e, provide the charge on a nucleus, so the
atomic number of an element is the same as the number of protons in the nuclei
of its atoms.
Ordinary matter, then, is mostly empty
space. The solid wood of a table, the steel that supports a bridge, the hard
rock underfoot, all are simply collections of tiny charged particles
comparatively farther away from one another than the sun is from the planets.
If all the actual matter, electrons and nuclei, in our bodies could somehow be
packed closely together, we would shrivel to specks just visible with a
microscope.
RUTHERFORD SCATTERING FORMULA
The formula that Rutherford obtained for
alpha particle scattering by a thin foil on the basis of the nuclear model of
the atom is
This formula is derived in the Appendix to
this chapter. The symbols in Eq. (4.1) have the following meanings:
N(θ) = number of alpha particles per unit
area that reach the screen at a scattering angle of θ
NI = total number of alpha
particles that reach the screen
n = number of atoms per unit volume in the
foil
Z = atomic number of the foil atoms
r = distance of the screen from the foil
KE = kinetic energy of the alpha particles
t = foil thickness
The predictions of Eq. (4.1) agreed with the
measurements of Geiger and Marsden, which supported the hypothesis of the
nuclear atom. This is why Rutherford is credited with the “discovery” of the
nucleus. Because N(θ) is inversely proportional to sin4 (θ/2) the
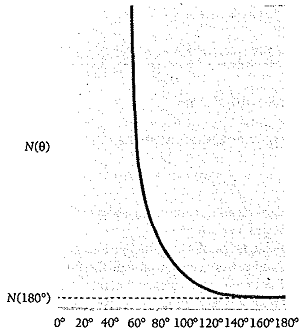
variation of N(θ) with θ is very pronounced (Fig. 4.4): only 0.14 percent of
the incident alpha particles are scattered by more than 1°.
Figure 4.4 Rutherford scattering. N(θ) is
the number of alpha particles per unit area that reach the screen at a
scattering angle of θ; N(180°) is
this number for backward scattering. The experimental findings follow this
curve, which is based on the nuclear model of the atom.
NUCLEAR DIMENSIONS
In his derivation of Eq. (4.1) Rutherford
assumed that the size of a target nucleus is small compared with the minimum
distance R to which incident alpha particles approach the nucleus before being
deflected away. Rutherford scattering therefore gives us a way to find an upper
limit to nuclear dimensions.
Let us see what the distance of closest
approach R was for the most energetic alpha particles employed in the early
experiments. An alpha particle will have its smallest R when it approaches a
nucleus head on, which will be followed by a 180° scattering. At the instant of
closest approach the initial kinetic energy KE of the particle is entirely
converted to electric potential energy, and so at that instant
since the charge of the alpha particle is 2e
and that of the nucleus is Ze. Hence
The maximum KE found in alpha particles of
natural origin is 7.7 MeV, which is 1.2 x 10-12 J. Since 1/4πεo,
= 9.0 x 109 N.m2/C2,
The atomic number of gold, a typical foil
material, is Z = 79, so that
The radius of the gold nucleus is therefore
less than 3.0 X 10-14 m, well under 10-4 the radius of
the atom as a whole.
In more recent years particles of much
higher energies than 7.7 MeV have been artificially accelerated, and it has
been found that the Rutherford scattering formula does indeed eventually fail
to agree with experiment. These experiments and the information they provide on
actual nuclear dimensions are discussed in Chap. 11. The radius of the gold
nucleus turns out to be about 1/5 of the value of R (Au) found above.
NEUTRON STARS
The
density of nuclear matter is about 2.4 X 1017 kg/m3,
which is equivalent to 4 billion tons per cubic inch. As discussed in Sec.
9.11, neutron stars are stars whose atoms have been so compressed that most of
their protons and electrons have fused into neutrons, which are the most stable
form of matter under enormous pressures. The densities of neutron stars are
comparable to those of nuclei: a neutron star packs the mass of one or two suns
into a sphere only about 10 km in radius. If the earth were this dense, it
would fit into a large apartment house.
Ernest
Rutherford (1871-4937), a native of New Zealand, was on his family’s farm
digging potatoes when he learned that he had won a scholarship for graduate
study at Cambridge University in England. “This is the last potato I will every
dig,“ he said, throwing down his spade. Thirteen years later he received the
Nobel Prize in chemistry.
At
Cambridge, Rutherford was a research student under J.J. Thomson, who would soon
announce the discovery of the electron. Rutherford’s own work was on the newly
found phenomenon of radioactivity, and he quickly distinguished between alpha
and beta particles, two of the emissions of radioactive materials. In 1898 he
went to McGill University in Canada, where he found that alpha particles are
the nuclei of helium atoms and that the radioactive decay of an element gives
rise to another element. Working with the chemist Frederick Soddy and others,
Rutherford traced the successive transformations of radioactive elements, such
as uranium and radium, until they end up as stable lead.
In
1907 Rutherford returned to England as professor of physics at Manchester,
where in 1911 he showed that the nuclear model of the atom was the only one
that could explain the observed scattering of alpha particles by thin metal
foils. Rutherford’s last important discovery, reported in 1919, was the
disintegration of nitrogen nuclei when bombarded with alpha particles, the
first example of the artificial transmutation of elements into other elements.
After other similar experiments, Rutherford suggested that all nuclei contain
hydrogen nuclei, which he called protons. He also proposed that a neutral
particle was present in nuclei as well.
In
1919 Rutherford became director of the Cavendish Laboratory at Cambridge, where
under his stimulus great strides in understanding the nucleus continued to be
made. James Chadwick discovered the neutron there in 1932. The Cavendish
Laboratory was the site of the first accelerator for producing high-energy
particles. With the help of this accelerator, fusion reactions in which light
nuclei unite to form heavier nuclei were observed for the first time.
Rutherford
was not infallible: only a few years before the discovery of fission and the
building of the first nuclear reactor, he dismissed the idea of practical uses
for nuclear energy as “moonshine.” He died in 1937 of complications of a hernia
and was buried near Newton in Westminster Abbey.










No comments:
Post a Comment